29+ calculate the ph of a buffer
A buffer maintains a relatively constant pH when acid or base is added to a solution. To calculate the specific pH of a given buffer you need to use the Henderson-Hasselbalch equation for acidic buffers.
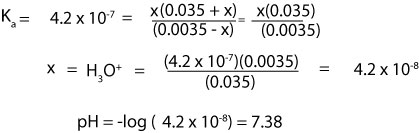
How Do You Calculate The Ph Of A Buffer Solution
Calculate the pH of the following two buffer solutions.

. Web 7243 pH p K a log A HA Equation 7243 is called the Henderson-Hasselbalch equation and is often used by chemists and biologists to calculate the pH of a buffer. Web To calculate the specific pH of a given buffer you need to use the Henderson-Hasselbalch equation for acidic buffers. For the buffer solution just starting out it was 933.
Ad Over 27000 video lessons and other resources youre guaranteed to find what you need. The pH is defined through the concentration of text H H ions in the solution. View the full answer.
Web Buffers are chemical compounds that maintain a consistent pH in a solution despite the addition of acids or bases to said solution. PH pKa log10 A-. Use the values given in the Henderson-Hasselbalch equation to solve for pH.
Web The pH of the solution is then calculated to be. Kb 18 x 10-5 2. Web You can calculate the pH of buffer solution in two ways.
Web What is the pH of the buffer with 020 M NH3 and 030 M NH4NO3. Web Predicting the pH of a Buffer. Web Determine the pH of a buffer solution that contains 0100 M hydrazoic acid pKa 460 p K a 460 and 0100 M lithium azide.
PH 1400 pOH 1400 log 97 10 4 1099. In this unbuffered solution addition of the base results in a significant rise in. PH -log.
PH pKa log10A-HA where Ka is the dissociation. Effect of Buffer Concentration on the Capacity of a Buffer. PH pKa logA-HA where pKa.
Web The pH of the benzoic acid weak acid is calculated using the formula as follows. What is the pH of a buffer consisting. PH pKa log10A-HA where Ka is the dissociation.
Web To calculate the pH of the buffer before and after the addition of HNO3 we need to use the Henderson-Hasselbalch equation. So lets compare that to the pH we got in the previous problem. Web The pH is a measure of the acidity or basicity of an aqueous solution.
PH of Solution Find the pH of the solution obtained. Web Steps for Calculating the pH of a Buffer Step 1. Preparation of acetate buffer solutions with different pH values 1.
Web The pH of a buffer solution may be calculated as follows. Web Science Chemistry Procedure I. PH pH is a measure of the acidity.
The addition of even. Therefore the pH of. PH - log C 6 H 5 COOH K a - log 0206510 - 5 - log 00036 244.
First you can use the acid dissociation constant expression and second you can use the Henderson. PH pKa log A-HA The Henderson-Hasselbalch equation enables determination of a buffer solutions pH when the pKa is known. P H p K a l o g n A n H A Where pK a dissociation constant of the acid nA initial number of moles of.
List the values you are given. Find the pH of a buffer solution that contains. Calculate the pH of the solution that results from.
Web You can use the Henderson-Hasselbalch equation on strong acids but well have a situation where A- HA. Given Molarity of CH3COOH 20 MMolarity of CH3CO. Web To calculate the specific pH of a given buffer you need to use the Henderson-Hasselbalch equation for acidic buffers.
However its unnecessary to calculate the pH of strong acid solution. Calculate the salt and acid volumes required to prepare 10 ml of the acetate. Web The pH is equal to 925 plus 12 which is equal to 937.

Calculate Ph Of Buffer Solution

How To Calculate The Ph Of A Buffer Solution Youtube
![]()
Step By Step Approach To Arterial Blood Gas Analysis
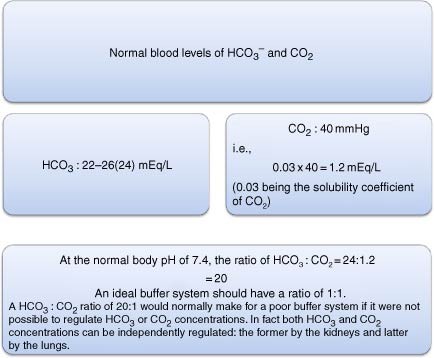
The Ph Springerlink

Chem 163 Chapter 19 Spring Buffers Solution That Resists Ph Changes Ex Blood Ph 7 4 Acid Must Neutralize Small Amounts Of Base Base Must Ppt Download
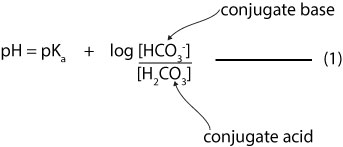
How Do You Calculate The Ph Of A Buffer Solution
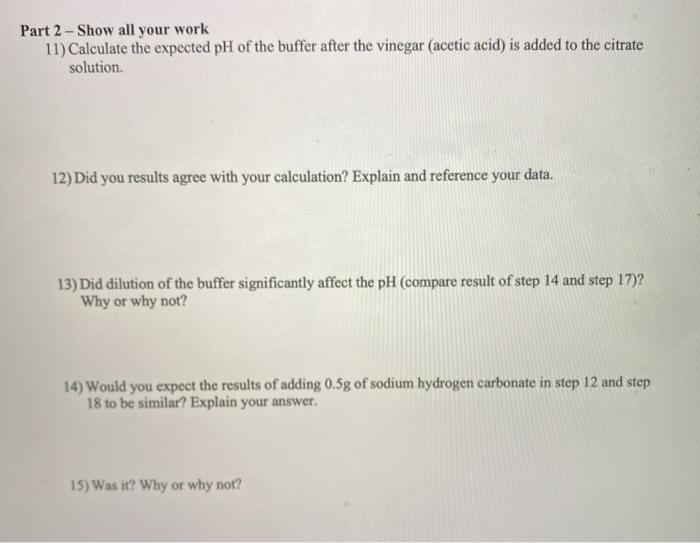
Part 2 Acetic Acid Acetate Ion Buffer 9 Prepare Chegg Com

How To Calculate Ph Of Buffer Solutions Sciencing

How To Calculate Ph Of Buffer Solutions Sciencing
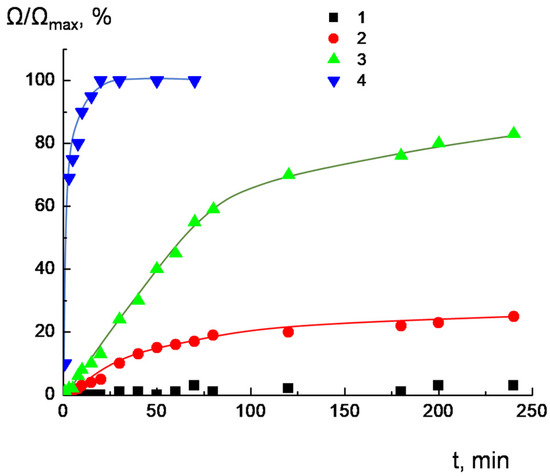
Ijms Free Full Text Complexes Of Cationic Pyridylphenylene Dendrimers With Anionic Liposomes The Role Of Dendrimer Composition In Membrane Structural Changes

Calculate Ph Of Buffer Solution
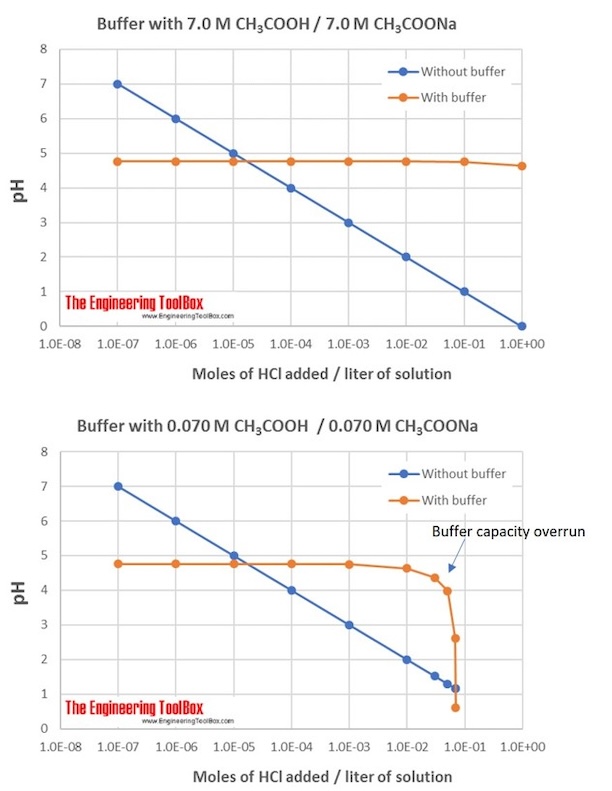
Buffer Solutions

Acid Base Why Is A Buffer Solution Best When Ph Pka I E When A Ha 1 Chemistry Stack Exchange
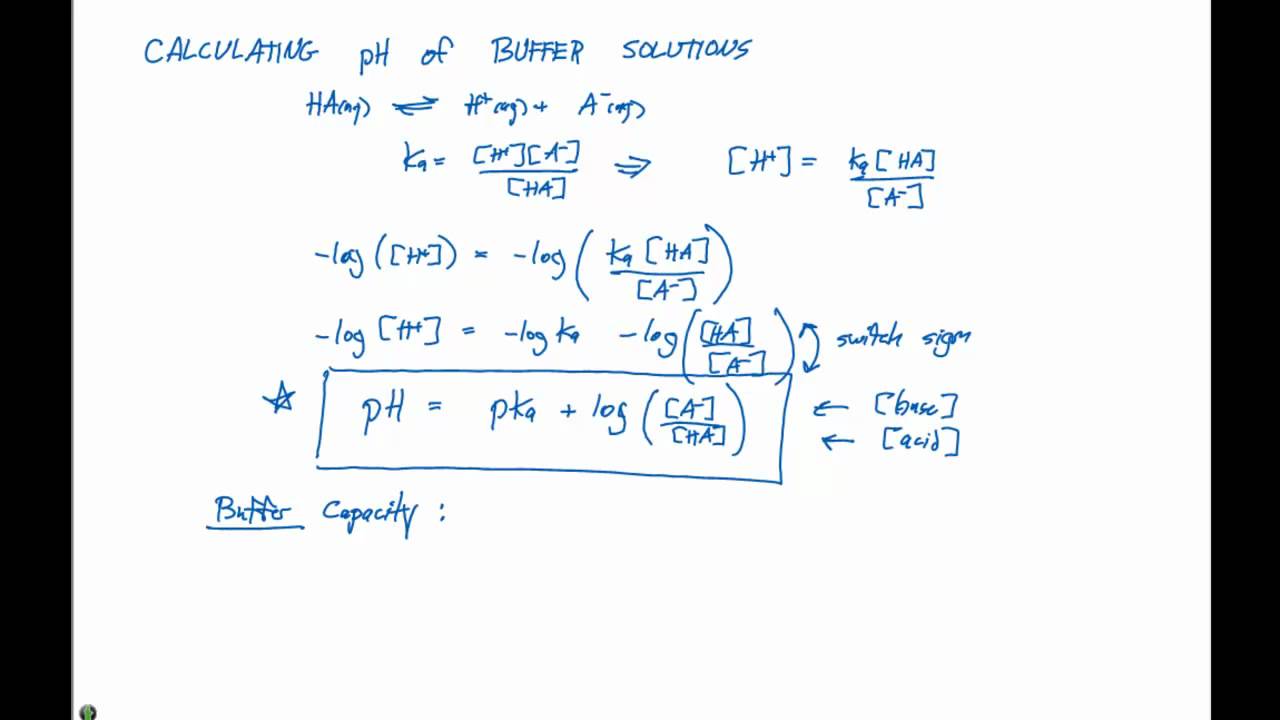
17 2 Calculating Ph Of Buffer Solutions Youtube
How To Calculate Ph Of Buffer Solutions Sciencing

17 6c Calculating The Ph Of A Buffer Youtube

1 How Much Does The Ph Of A Buffer Change When An Acid Or Base Is Added Though Buffers Do Resist Change In Ph When Acid Or Base Are Added To